Soil samples should be obtained at least every three to four years using sampling techniques recommended by local University Extension offices. Soil sampling should occur after harvest or before planting in the spring. Fall sampling is preferred for determining lime application recommendations. However, it can be a challenge to collect soil samples in the fall because of workload, soil moisture content, a late harvest, or other reasons. These reasons can make it difficult to have sampling results available in time for fall applications. As an alternative, fall fertilizer recommendations can be based on samples collected the previous spring providing the recently harvested crop’s nutrient removal is considered.
Sampling recommendations may differ for farming operations using grid sampling. General recommendation is to soil sample for phosphorus (P) every five years. Since lime applications can last 8 to 10 years, soil sampling for lime after 8 years is recommended.
Nutrient removal varies depending on soil conditions, yields, product differences, and fertilizer rates. Soil tests can estimate the fertilizer quantity required based on crop yield.
Lime Application
Lime is required to neutralize soil acidity by altering soil pH. Soil pH can range from 4.0 (strongly acid) to 10.0 (strongly alkaline) and nutrients become more, or less available depending on pH. Nutrients are most available to plants when soil pH ranges between 6.0 and 7.0. Lime takes time to dissolve and neutralize acidity; therefore, it should be applied three to six months before planting crops. Additionally, different lime sources because of particle size dissolve quicker and have different neutralizing efficiencies. Because lime can interfere with the availability of nutrients, especially phosphorus (P), lime should be applied and incorporated at least a month before applying other nutrients. Sources of lime include, a material containing calcium (Ca) and/or magnesium (Mg) that are capable of neutralizing soil acidity. These materials include limestone (both calcitic and dolomitic), burnt lime, slaked lime, marl, and various by-products.
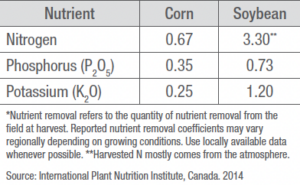
Phosphorus and Potassium
Phosphorus (P) and potassium (K) are typically applied in the fall because of generally dry soils, availability of fertilizers, workload, and tillage requirement to help reduce loss through erosion or runoff. Applications of P and K to frozen soils should be avoided because potential runoff can impact surface water leading to algae blooms. Common P fertilizers include superphosphate, concentrated superphosphate (CSP), monoammonium phosphate (MAP), diammonium phosphate (DAP), and ammonium polyphosphate (APP). Common K fertilizers include potassium chloride, potassium sulfate, potassium-magnesium sulfate, potassium thiosulfate, and potassium nitrate.
In the soil, P is very immobile and held tightly by soil colloids. Crop roots must be able to reach P soil concentrations for uptake. Any stress that reduces root growth can result in plants showing P deficiency. Potassium is either available, slowly available, or readily available depending on the parent material holding the K and how quickly the parent material releases K through weathering. The amount of K leaching is generally low unless it becomes part of the soil solution and is leached.
It is common to apply P and K fertilizers on a two-year cycle of crop production, usually after a soybean crop, to maintain soil P and K fertility. This is particularly true for K as a soybean crop requires a substantial amount of K. Corn residue, if not removed, can return approximately 60% of the K utilized by the plant during growth. Soil tests should be conducted to determine if P and K levels have dropped below maintenance levels which would require higher application rates to restore adequate soil reserves.
Nitrogen Application
Reduced soil compaction, workload distribution, and economic incentives are advantages for applying nitrogen (N) in the fall. Disadvantages can include the loss of N between application and crop use and environmental concerns of nitrates leaching into streams, lakes, and groundwater.
Fertilizers comprised predominantly of ammonium-N are preferred for fall application. Nitrogen sources containing urea and/or nitrates have greater potential to be lost via leaching, volatilization, or runoff. Nitrate-N is subject to loss via leaching and denitrification while ammonium-N is relatively stable as it attaches to soil particles. Due to these concerns, anhydrous ammonia is a good source for fall-applied N.
Anhydrous ammonia should be applied in late fall after soil temperatures cool to 50° F or less and cool weather is expected to continue (Figure 1). Generally, fall N applications are only feasible in areas where winter soil temperatures limit ammonium nitrification. It should not be applied in areas where soils seldom or never freeze or where there is a long period between the time soils reach 50° F and when they freeze. Nitrifying organisms that convert ammonium to nitrates can continue to function at temperatures as low as 32° F; however, the rate of nitrification is reduced. Long periods at soil temperatures above 32° F can still result in nitrification of a large percentage of the applied ammonium-N. The use of a nitrification in late fall after soils cool to 50° F. inhibitor can slow the conversion of ammonium to nitrates and improve the effectiveness of fall-applied N. Avoiding fall N applications to sandy and poorly drained soils is a best management practice.
Splitting N requirements between fall, spring, and sidedress should be considered. Spring and sidedress applications may allow for increased N utilization, particularly when growing conditions are wet and cool. The Corn Nitrogen Rate Calculator developed by Iowa State University in association with other Midwestern Universities is a tool that can help find the maximum return to N and the most profitable N rate.

Figure 1. Anhydrous ammonia should be applied in late fall after soils cool to 50° F.
Manure Application
Manure can be an adequate nutrient source of N, P, K, and micronutrients. However, one application may not be optimal for all nutrients. The nutrient content of manure varies depending on livestock type, feed ration, and manure storage and handling. A manure nutrient analysis should be completed prior to application. Manure sources that have high inorganic ammonium content, such as liquid swine manure, should be applied in late fall after soils cool. For manure with considerable bedding, fall application can provide a longer time-period for microbial mineralization to inorganic N which can increase N availability to the crop.
Manure injection or incorporation should be considered to reduce potential N volatilization and P runoff. Manure applications to frozen or sloping soils and to residue-free fields should be avoided. Cover crops can help retain N and P in no-till cropping systems.
