Key Points
- High soil pH can cause nutrient deficiencies in plants, which can result in lost yield potential.
- Saline and sodic soils can cause osmotic stress and ion toxicity in plants, and sodic soils can negatively impact soil structure.
- Management of these soil issues requires different approaches, which will vary by region and soil type.
Salinity, sodicity, and high pH in soils can impact plant growth and yield potential. These issues are common in the western growing regions of the US, such as the Great Plains, and are primarily caused by the weathering or breakdown of soil parent material or by the use of poor-quality irrigation water.
High pH, Salinity, and Sodicity
Soil pH is a measure of the acidity (low pH) or alkalinity (high pH) of the soil. The pH value for good corn production is between 6.0 to 6.5. High pH problems are associated with a pH 7.8 or higher and are often accompanied by saline soils, sodic soils, or saline-sodic soils. Problems with soil pH
(either too high or too low) can cause the following issues in plants:
- Inadequate pH levels can result in certain nutrients becoming tightly bound to the soil or precipitating out of the soil solution into solid materials, making them unavailable to the plant. Alternatively, other nutrients can become increasingly available, resulting in toxic levels in the plant. For example, calcium availability can be substantially reduced and manganese can become toxic at a low pH, while at high pH levels most micronutrients become unavailable and calcium levels can be substantially elevated (Figure 1). Phosphorus can become tied up at both a low and high pH.
- Inadequate pH levels can influence the activity of herbicides and other chemical reactions, as well as microbial activity. For example, low pH decreases the activity of nitrifying bacteria that convert ammonium to nitrate, the predominant form used by plants.
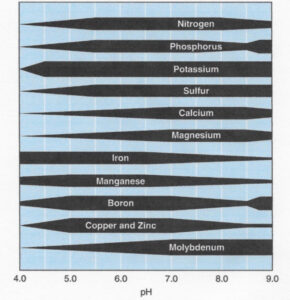
Figure 1. The relationship between soil pH and nutrient availability. The wider the dark bar, the greater the nutrient availability. Image courtesy of Fernández, F.G. and Hoeft, R.G. Managing soil pH and crop nutrients. Chapter 8. Illinois Agronomy Handbook. University of Illinois.
Soil salinity is the content of soluble salts in the soil, which can readily dissolve in the soil water solution and be taken up by plants. These salt ions include sodium (Na+), potassium (K+), magnesium (Mg2+), calcium (Ca2+), chloride (Cl- ), sulfate (SO42-), carbonate (CO32-), bicarbonate (HCO3- ), and nitrate (NO3- ).
Soil sodicity is the concentration of exchangeable sodium ions in the soil. Sodic soils have high levels of sodium and low levels of other salts. Sodic soils can have structural issues because the sodium ions weaken soil aggregates, resulting in a collapse of the soil structure. This is especially common in sodic soils with high clay or silt content. Sodic soils often have a high pH, often greater than 8.4.
Some soils may have both high saline and high sodium levels; these are termed saline-sodic soils. When salts build up in the soil, the following issues can occur in plants:
- High levels of salts in the soil water solution can cause osmotic stress to the plant. This is because water will move from a less salty solution (generally the soil) to a more salty solution (generally the plant cell). The potential for water to move into plant cells is reduced when the soil water solution also has a high salt concentration. Thus, in highly saline soils the plant cannot absorb water as efficiently.
- High levels of sodium and/or chloride can cause ion toxicity—which damages plant cells—and can interfere with the uptake and transport of essential nutrients, leading to nutrient deficiencies. These conditions can result in growth abnormalities, stunting, poor kernel set, reduced grain weight, and crop failure.
- The deterioration of the physical soil structure (known as deflocculation) in sodic soils can result in restricted water and air movement through the soil, which can cause difficulties with water penetration into the soil, soil crusting, and waterlogging.
- Germination and early seedling growth are the most sensitive growth stages to salt stress. Soil salinity and sodicity issues can occur anywhere, but are most common in areas with limited rainfall and high evapotranspiration, such as in arid and semi-arid growing regions, where the solutes are less likely to be leached through the soil profile and collect in the root zone. Irrigation water that contains high levels of solutes and high water tables that carry the solutes to the surface can also contribute to soil salinity and sodicity problems.
Identifying and Managing Salt and pH Issues:
- Nutrient deficiency symptoms may indicate pH issues in the soil. Zinc, iron, and phosphorus become less available to plants at high pH. Symptoms of phosphorus deficiency are a dark green or purple coloration of the leaves and stems. Zinc and iron deficiency symptoms are interveinal chlorosis (chlorotic stripes) on the leaves of the upper canopy. High levels of salts in the soil may also cause nutrient deficiencies.
- Frequent signs of water stress, such as wilting and leaf rolling, may indicate a problem with high salts. A white crust of excess salts may be visible on the surface of the soil.
- Severely sodic soils may have a dusty black residue on the surface, which is soil organic matter that has been released from the fractured soil aggregates.
Management options for high pH soils:
- Grow crops that are more tolerant to alkaline soils.
- Improve plant-available nutrients by adding fertilizers or chelates to the soil. A heavier reliance on starter fertilizers may be needed.
- If the soil does not contain free carbonates, adding elemental sulfur—which is turned into sulfuric acid by bacteria in the soil—or directly adding sulfuric acid may help to acidify the soil over time. However, this is usually uneconomical for large acres as the amount of sulfur required to lower the pH may be exceptionally high.
- Adding organic matter in the form of manures or crop residue can lower the pH over time as the breakdown of organic matter releases acids while also providing essential nutrients, including micronutrients.
Note that it may be more difficult to lower the pH of high pH soils than to manage their soil nutrient availabilities. This is because high pH is often caused by the parent material of the soil, which will continue to break down over time and buffer any attempts to acidify the soil. Soils that contain free carbonates are the most difficult to alter in terms of pH, though the addition of thiosulfate to a 2 x 2 starter maybe helpful.
Management options for saline soils:
- Switch to a salt-tolerant corn product or a different salt-tolerant crop.
- An irrigation application with excess water (over-irrigation) can help to leach some of the salts below the root zone. This can be done prior to planting to reduce salt stress on seedlings, post-harvest to avoid leaching of fertilizer nutrients, or when high-quality irrigation water is most available. Fields with shallow water tables may require the installation of artificial drainage prior to leaching.
- Increase irrigation frequency so that soils do not dry as much between irrigations and salt concentrations remain diluted.
- Improve residue management to minimize evaporation, which can reduce the amount of salts that are pulled up from below the root zone.
Management options for sodic soils:
- Switch to a sodium-tolerant corn product or a different sodium-tolerant crop.
- The excess sodium ions can be removed from the soil by increasing the concentration of calcium ions. Soil particles will swap one cation for another depending on the concentration of the different cations in the soil. At higher Ca2+ concentrations, the Na+ bound to the soil will be replaced by the Ca2+, allowing the free Na+ ions to be leached with an over-irrigation. This is accomplished by dissolving the limestone
(calcium carbonate) or gypsum (calcium sulfate) in the soil with elemental sulfur or sulfuric acid, which releases the calcium. If the soil does not contain lime or gypsum, calcium can be added directly to the soil. Then the sodium is leached from the soil with an over-irrigation. - Use management practices that help to restore soil structure such as residue management and adding organic matter to the soil with manure or cover crops.
Note that the practice of leaching the salts from the soil may also remove soil nutrients and pesticides, and will reduce irrigation efficiency. Consider the soil fertility level (fall may be the best time as nutrients have already been utilized by the plant), drainage, the quality of the irrigation water at the time of the leaching event, the availability of the irrigation water, and the type of irrigation system (to ensure the irrigation capacity is sufficient to apply enough water in a short enough of time to cause leaching).
